Hammerschmidt Group - Infection Biology
Infections with pathogens cause over 25% of global deaths. Prof. Hammerschmidt's research group investigates the molecular mechanisms of bacterial infections and the mechanisms of the immune defense against Gram-positive bacteria.
Molecular and cellular Infection Biology: Streptococcus pneumoniae
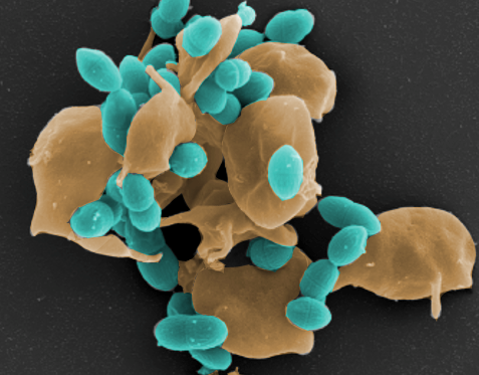
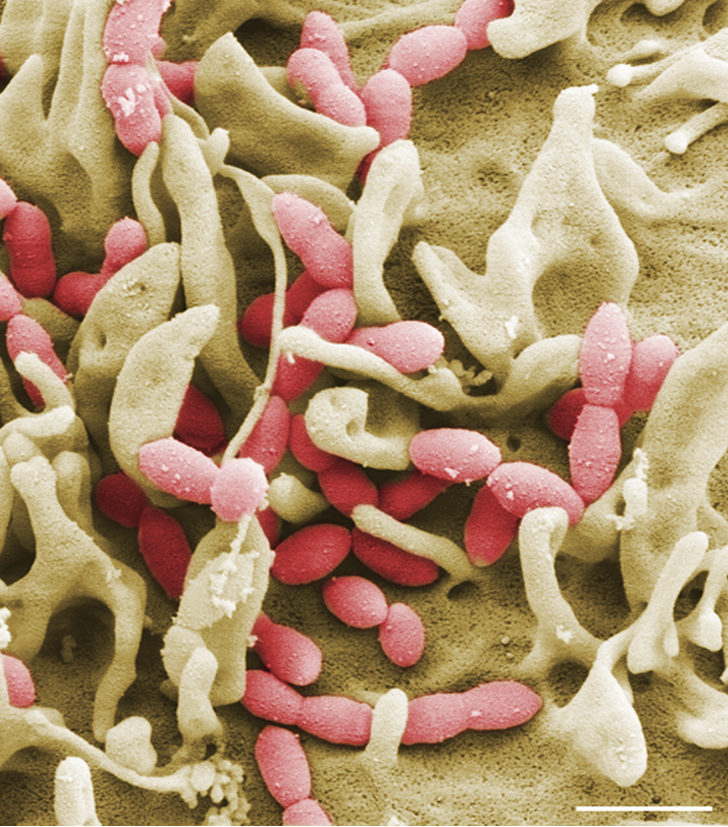
Streptococcus pneumoniae (pneumococci) are Gram-positive bacteria and belong to the normal flora of the respiratory tract in healthy adults and children. On the other hand, pneumococci are the causative agents of severe local inflammation and respiratory diseases. The spread of pneumococci in humans causes life-threatening diseases in addition to otitis media. Pneumococci are the most common causative agents of community-acquired pneumonia (AEP) and common causative agents of bacterial meningitis and sepsis. The mechanisms and factors that favor a change from a commensal to a pathogenic bacterium are little researched. In our projects, we want to identify the bacterial factors involved in the pathogenesis of pneumococcal diseases. Furthermore, we want to investigate the molecular mechanisms of pathogen-host interaction in infections in cell culture and under physiological and in vivo relevant conditions.
Current Research Topics
Pneumococal adaptation under in vivo conditions
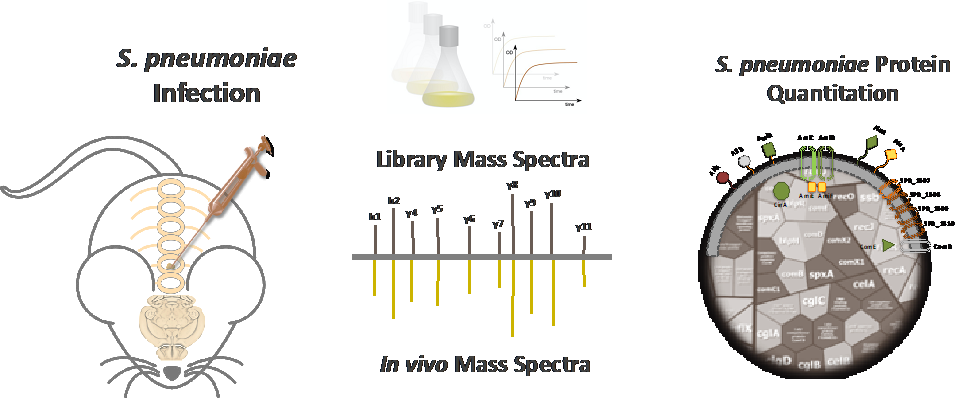
A hallmark of pneumococci is their versatility. While on the one hand colonizing as harmless commensals the upper respiratory tract, pneumococci can on the other hand cause severe local and invasive diseases including pneumonia, sepsis, and meningitis. To identify host compartment specific expressed pneumococcal proteins, we have established mouse models and a work flow for an in vivo proteome-based approach. This strategy will decipher the pneumococcal physiology and the key fitness as well as virulence factors within defined host compartments. In turn, compartment-specific proteins might represent promising candidates for protein-based vaccines.
The interplay between platelets and pathogenic bacteria
Platelets modulate bacterial infections. We are interested in the interplay between platelets and pathogenic bacteria such as Staphylococcus aureus and Streptococcus pneumoniae. Therefore, this project aims to identify bacterial factors acting on platelets, as well as studying key platelet receptors involved at the platelet bacteria interface. We have established a repertoire of S. aureus and His6-tagged pneumococcal proteins, purified cell wall components and various platelet function assays, allowing even single cell analysis. All these assays will initially be conducted in established in vitro experiments using human platelets (Funded by DFG-CRC Transregio 240, project A11.
Pneumococci at the host-pathogen interface
Colonization of mucosal respiratory surfaces is a prerequisite for the pneumococcus to cause severe invasive infections. The arsenal of pneumococcal adhesins such as PavB, PspC, enolase, PsrP, interacts with a multitude of extracellular matrix (ECM) as well as serum proteins including Factor H (FH), fibronectin, plasmin(ogen), laminin, thrombospondin-1, secretory IgA, vitronectin, von Willebrand factor (vWF). In addition, pneumococcal adhesin bind also directly to host cellular receptors such as the polymeric Ig receptor (pIgR). Since the diverse interactions of pneumococci with the ECM or serum proteins facilitate colonization and immune evasion, their molecular characterization not only allows a better understanding of microbial invasion but also provides clues. for the design of noel therapeutic strategies to manage infectious diseases.
Regulation of pneumococcal gene expression
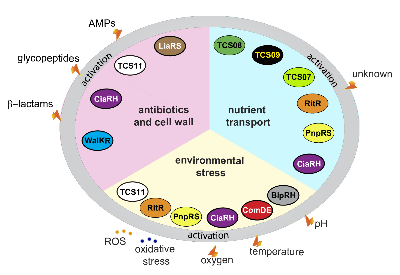
Pneumococcal pathophysiological processes are regulated by stand-alone regulators or two-component regulatory systems (TCS). The diverse array of fitness and virulence factors expressed by pneumococci have to be tightly controlled at the transcriptional level. Our projects aim to elucidate the function of stand-alone regulators such as ArgR2 involved in the regulation of the arginine-deiminase system and TCS such as TCS08 and TCS09. In addition, workflows are established to identify host compartment specific gene expression signatures by in vivo RNAseq.
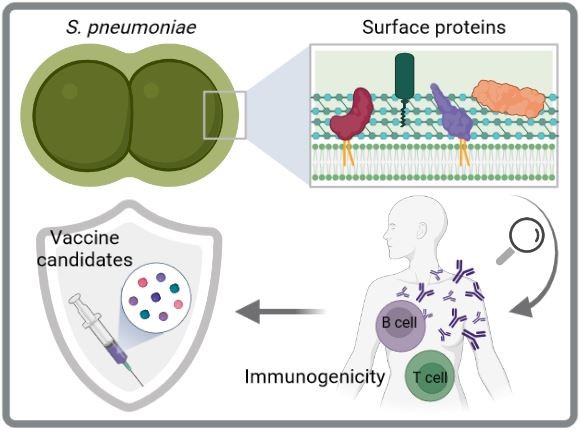
Immunogenicity and protectivity of pneumococcal surface proteins
Current pneumococcal vaccines are an important means of controlling infections caused by S. pneumoniae. However, they have significant limitations including restricted serotype coverage, which facilitates replacement by non-vaccine serotypes, and high manufacturing costs. Therefore, serotype-independent and protein-based next-generation vaccines are preferred for future vaccine strategies. The cell surface of pneumococci is decorated with several protein clusters including choline-binding proteins, sortase-anchored proteins, and lipoproteins. Here, we aim to decipher the immunogenicity and protective potential of surface-exposed pneumococcal proteins through various in vitro and in vivo studies to identify suitable vaccine candidates. Especially lipoproteins are interesting candidates as they are highly conserved, abundant and immunogenic.
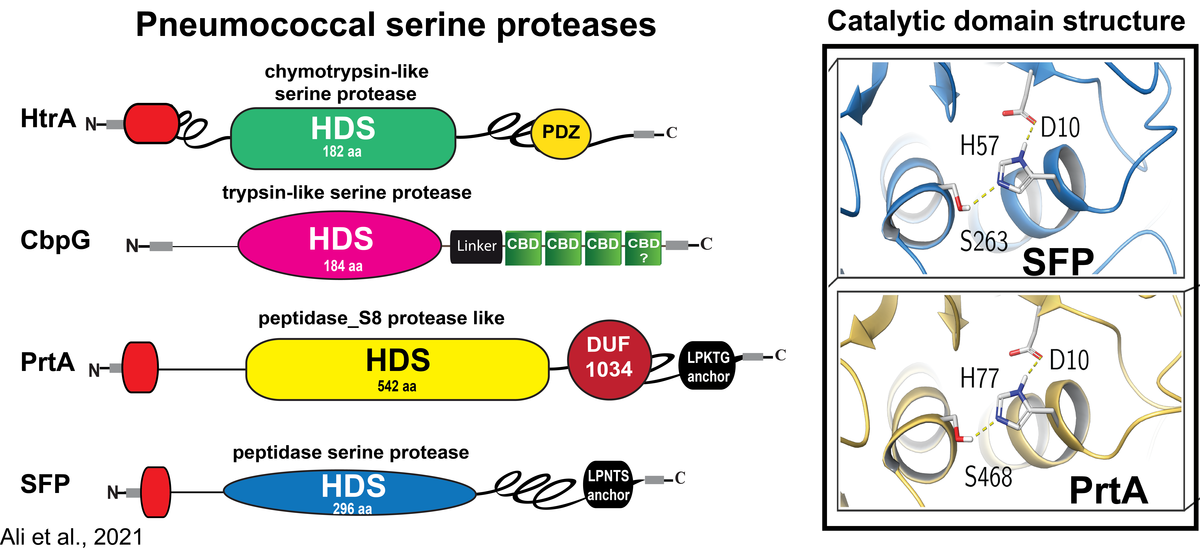
Role of serine proteases in the pathophysiology of Streptococcus pneumoniae
Pneumococci colonize as a harmless commensal the upper respiratory tract of human. When transmigrating to the lung pneumococci can breach under certain physiological and immunological conditions the epithelial barrier and cause pneumonia and severe invasive diseases. We and others have shown that colonization is facilitated by direct interactions with host cell receptors or via binding to components of the extracellular matrix (ECM) bound to host cell receptors. Similar, pneumococci engage the ECM and serum proteins to evade the innate immune system. To breach the epithelial barrier pneumococci and other bacteria abuse host-derived extracellular proteases or exploit endogenous enzymes and surface-exposed proteases for tissue remodeling processes. We have shown earlier that pneumococci hijack host-derived proteolytic activities like the serine protease plasmin(ogen) to degrade the extracellular matrix, to colonize and invade host tissues. In contrast, the role of pneumococcal serine proteases containing the typical Asp-Ser- His triad essential for their catalytic activity in colonization, mucus degradation, or epithelial barrier disruption is insufficiently explored.
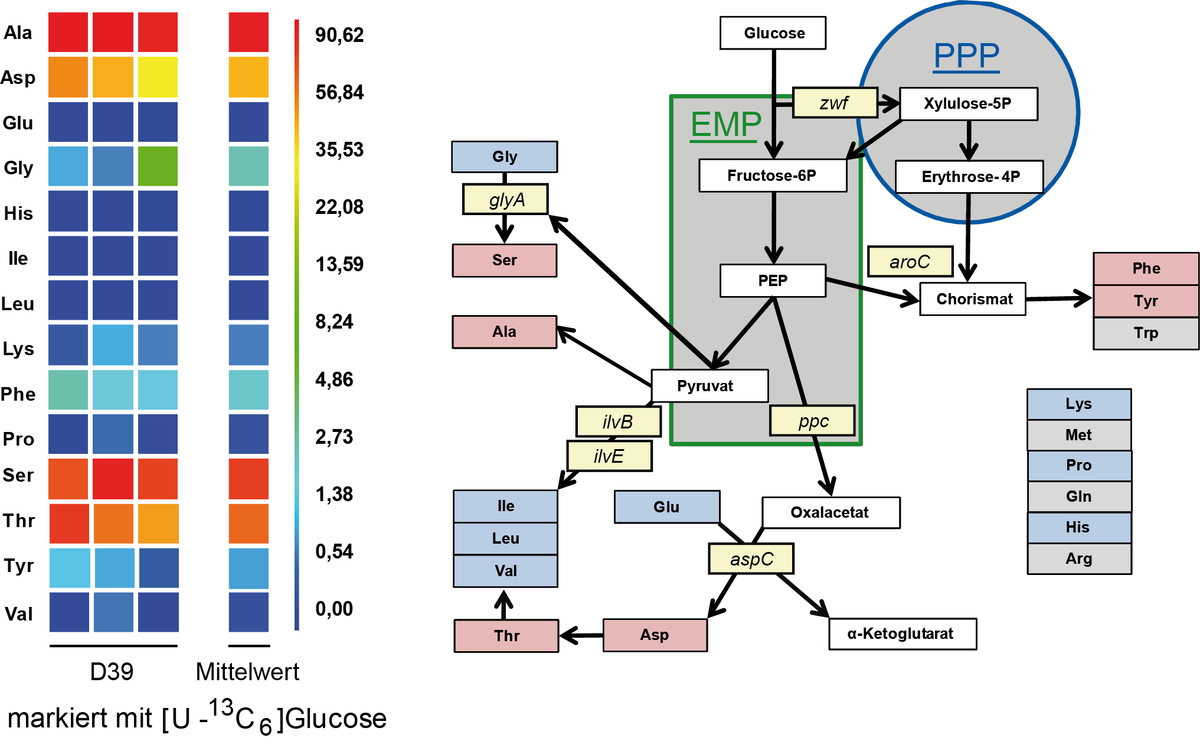
Studies on carbon and N-metabolism in Streptococcus pneumoniae
Streptococcus pneumonia is able to metabolize different sugars like glucose, galactose or lactose as carbon source under facultative anaerobic conditions. Under fermentative conditions the main product lactate is formed. Galactose is an important intermediate necessary for capsule biosynthesis. Carbon metabolism was studied with 13C-labeled glucose and isotopoloque profiling and GC/MS analyses were performed. It was demonstrated for strain D39 that synthesis from serine is made out of glycine by the action of serine hydroxymethyltransferase reaction. For further studies 15N labeled amino acids will be used to estimate the uptake and usage as nitrogen source in different pneumococcal strains.
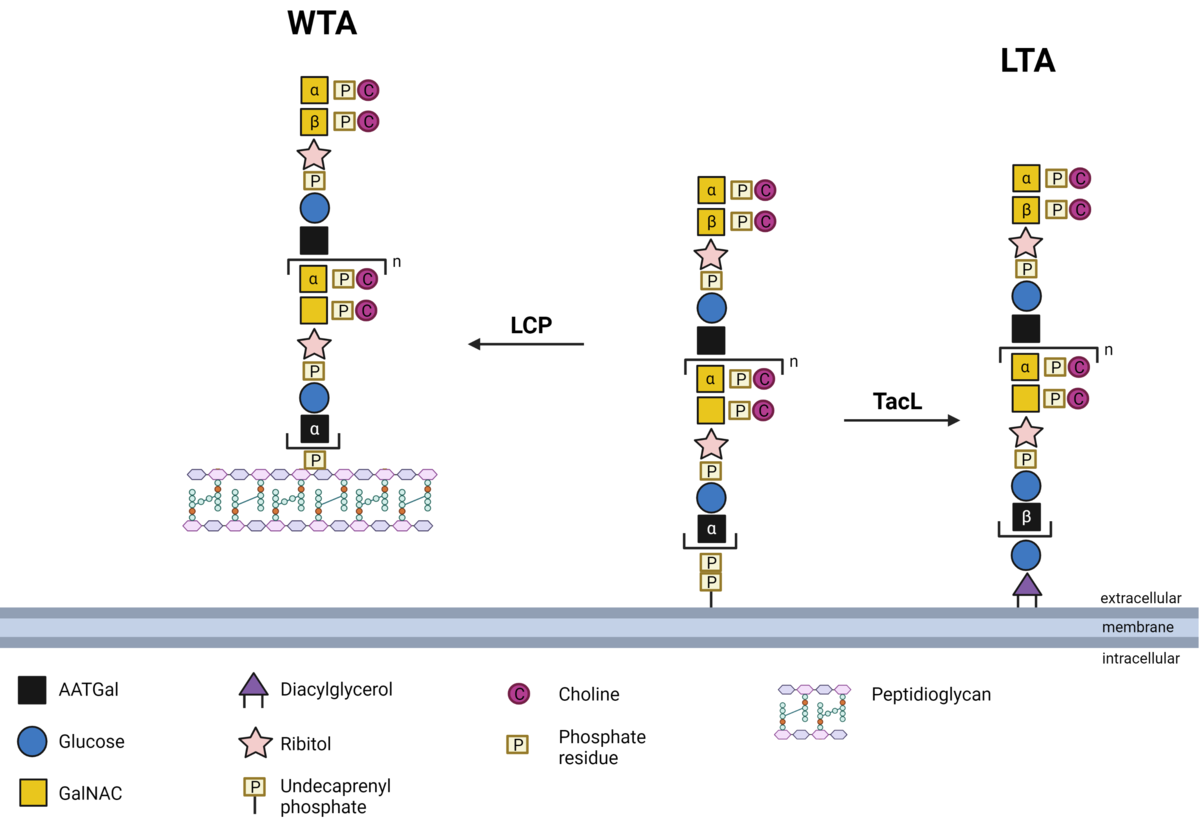
Biosynthesis of pneumococcal teichoic acids
Teichoic acids are essential cell wall components of Gram-positive bacteria and hence, also from pneumococci. These are sugar polymers consisting of homopolymeric repeating units (RUs) are either bound to the peptidoglycan as wall teichoic acid (WTA) or anchored in the membrane as lipoteichoic acid (LTA). Pneumococcal teichoic acids are substituted with phosphorylcholine (PCho) and the so-called choline-binding proteins (CBPs) are bound non-covalently to the teichoic acids. In recent studies we have deciphered the anchoring of lipoteichoic acids in the bacterial cell membrane via the teichoic acid ligase (TacL). Our ongoing research focuses e.g.,, on the identification of proteins catalyzing the anchoring of wall teichoic acids in the peptidoglycan. Structural chemical analyses of generated bacterial deletion mutants as well as the quantification of the teichoic acids are conducted in collaboration with the Research Center Borstel, Leibniz Lung Center (Dr. Nicolas Gisch). PI: Prof. Hammerschmidt, doctoral researcher: Max Brendel (Dipl. Pharm), Co-supervision: Dr. Thomas Kohler (DFG HA 3125/5-2)
Current Third-party Funding
- DFG | German Research Foundation
- BMBF | Federal Ministry of Education and Research - InfectControl 2020
- Federal State of Mecklenburg-Vorpommern | KoInfekt
- DAAD | German Academic Exchange Service
- IB-ASIEN | SOUTHEAST ASIA EUROPE
Cooperations
- Prof. Dr. Juan Hermoso, CSIC, Madrid, Spain
- Prof. Dr. Uwe Koedel, Neurologie, LMU Munich
- Prof. Dr. Manfred Rohde, Helmholtz-Zentrum für Infektionsforschung, Braunschweig
- Prof. Dr. Jan Maarten van Dijl, Groningen, Netherlands
- Prof. Dr. Marien DeJonge, Nijmegen, Netherlands
- Dr. Nicolas Gisch, Research Center Borstel
- Prof. Peter F. Zipfel, Hans-Knöll Institut, Jena
- PD Dr. Wolfgang Eisenreich, Biochemie, TU Munich
- Prof. Dr. Gustavo Gámez, Universidad de Antioquia, Medellín, Colombia
- Prof. Stefan Hippenstiel, Prof. Andreas Hocke, Prof. Leif Sander, Prof. Norbert Suttorp, Department of Internal Medicine, Infectious and Respiratory Diseases, University Medicine Berlin