AG Hammerschmidt - Infektionsbiologie
Infektionen mit pathogenen Erregern verursachen über 25 % der weltweiten Todesfälle. Die Arbeitsgruppe von Prof. Hammerschmidt erforscht die molekularen Mechanismen bakterieller Infektionen und die Mechanismen der Immunabwehr Gram-positiver Bakterien.
Molekulare und zelluläre Infektionsbiologie: Streptococcus pneumoniae
Streptococcus pneumoniae (Pneumokokken) sind Gram-positive Bakterien und gehören zur Normalflora des Respirationstraktes bei gesunden Erwachsenen und Kindern. Auf der anderen Seite sind Pneumokokken die Erreger schwerer lokaler Entzündungen und respiratorischer Erkrankungen. Die Ausbreitung der Pneumokokken im Menschen verursacht neben der Mittelohrentzündung auch lebensbedrohliche Erkrankungen. So sind Pneumokokken die häufigsten Erreger einer ambulant erworbenen Pneumonie (AEP) und häufige Erreger einer bakteriellen Meningitis und Sepsis. Die Mechanismen und Faktoren, die eine Umwandlung vom Kommensalen in ein pathogenes Bakterium begünstigen, sind wenig erforscht. Wir wollen in unseren Projekten die bakteriellen Faktoren identifizieren, die an der Pathogenese der Pneumokokken-Erkrankungen beteiligt sind. Des Weiteren wollen wir die molekularen Mechanismen der Erreger-Wirt-Interaktion in Infektionen in der Zellkultur und unter physiologischen und in vivo relevanten Bedingungen untersuchen.
Spezifische Forschungsschwerpunkte
Die Anpassung von Pneumokokken unter in vivo Bedingungen
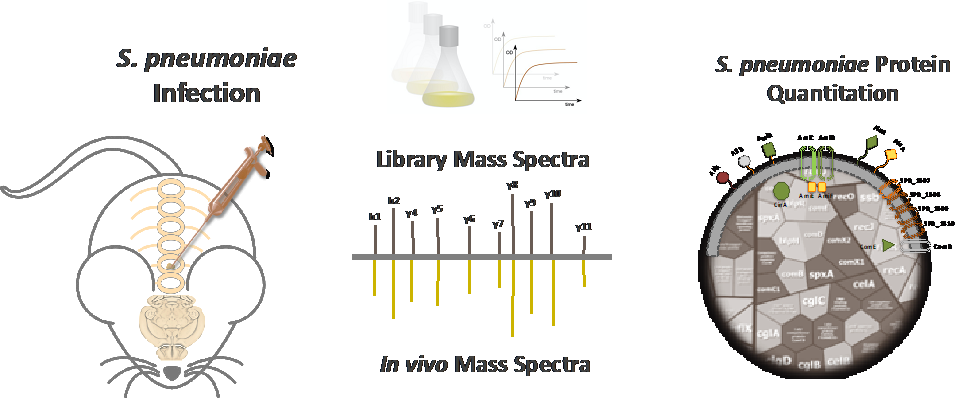
Ein Markenzeichen der Pneumokokken ist ihre Vielseitigkeit. Während auf der einen Seite als harmlose Kommensalen die oberen Atemwege besiedelt werden, können Pneumokokken auf der anderen Seite schwere lokale und invasive Erkrankungen wie Lungenentzündung, Sepsis und Meningitis verursachen. Um wirtskompartimentspezifisch exprimierte Pneumokokkenproteine zu identifizieren, haben wir Mausmodelle und einen Workflow für einen in vivo Proteom-basierten Ansatz etabliert. Diese Strategie wird die Physiologie der Pneumokokken und die wichtigsten Fitness- und Virulenzfaktoren innerhalb definierter Wirtskompartimente entschlüsseln. Im Gegenzug könnten kompartimentspezifische Proteine vielversprechende Kandidaten für proteinbasierte Impfstoffe darstellen.
Das Zusammenspiel von Blutplättchen und pathogenen Bakterien
Blutplättchen modulieren bakterielle Infektionen. Wir interessieren uns für das Zusammenspiel von Blutplättchen und pathogenen Bakterien wie Staphylococcus aureus und Streptococcus pneumoniae. Daher zielt dieses Projekt darauf ab, bakterielle Faktoren zu identifizieren, die auf Blutplättchen wirken, sowie wichtige Thrombozytenrezeptoren zu untersuchen, die an der Thrombozytenbakterien-Grenzfläche beteiligt sind. Wir haben ein Repertoire an S. aureus- und His6-markierten Pneumokokkenproteinen, gereinigten Zellwandkomponenten und verschiedenen Thrombozytenfunktionsassays etabliert, die sogar einzellige Analysen ermöglichen. Alle diese Assays werden zunächst in etablierten In-vitro-Experimenten mit menschlichen Blutplättchen durchgeführt (Gefördert durch DFG-SFB Transregio 240, Teilprojekt A11).
Pneumokokken an der Wirt-Pathogen-Grenzfläche
Die Besiedlung der Atemwegsschleimhäute ist eine Voraussetzung dafür, dass Pneumokokken schwere invasive Infektionen verursachen können. Das Arsenal an Pneumokokken-Adhäsinen wie PavB, PspC, Enolase, PsrP interagiert mit einer Vielzahl von extrazellulären Matrix-(ECM) sowie Serumproteinen einschließlich Faktor H (FH), Fibronektin, Plasmin(ogen), Laminin, Thrombospondin-1, sekretorischem IgA, Vitronektin, von Willebrand-Faktor (vWF). Darüber hinaus bindet Pneumokokken-Adhäsin auch direkt an zelluläre Wirtsrezeptoren wie den polymeren Ig-Rezeptor (pIgR). Da die vielfältigen Wechselwirkungen von Pneumokokken mit dem ECM oder Serumproteinen die Besiedlung und Immunvermeidung erleichtern, ermöglicht ihre molekulare Charakterisierung nicht nur ein besseres Verständnis der mikrobiellen Invasion, sondern liefert auch Hinweise für die Entwicklung von neuen therapeutischen Strategien zur Behandlung von Infektionskrankheiten.
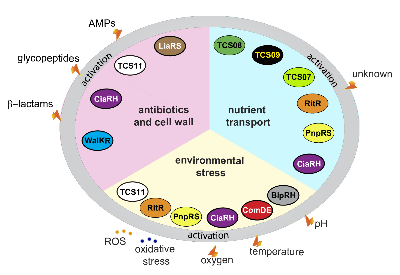
Regulation der Pneumokokken-Genexpression
Pneumokokken-pathophysiologische Prozesse werden durch eigenständige Regulatoren oder Zwei-Komponenten-Regulationssysteme (TCS) reguliert. Die vielfältigen Fitness- und Virulenzfaktoren, die von Pneumokokken exprimiert werden, müssen auf transkriptioneller Ebene streng kontrolliert werden. Unsere Projekte zielen darauf ab, die Funktion von Stand-alone-Regulatoren wie ArgR2, die an der Regulierung des Arginin-Deiminase-Systems und TCS wie TCS08 und TCS09 beteiligt sind, aufzuklären. Darüber hinaus werden Workflows eingerichtet, um wirtskompartimentspezifische Genexpressionssignaturen durch in vivo RNAseq zu identifizieren.
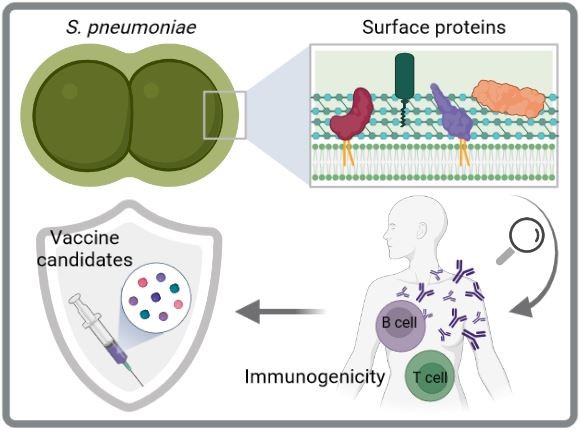
Immunogenität und Schutzfähigkeit von Pneumokokken-Oberflächenproteinen
Aktuelle Pneumokokken-Impfstoffe sind ein wichtiges Mittel zur Kontrolle von Infektionen, die durch S. pneumoniae verursacht werden. Sie haben jedoch erhebliche Einschränkungen, einschließlich einer eingeschränkten Serotypabdeckung, die den Ersatz durch Nicht-Impfstoff-Serotypen erleichtert, und hohe Herstellungskosten. Daher werden serotypunabhängige und proteinbasierte Impfstoffe der nächsten Generation für zukünftige Impfstoffstrategien bevorzugt. Die Zelloberfläche von Pneumokokken ist mit mehreren Proteinclustern dekoriert, darunter Cholin-bindende Proteine, Sortase-verankerte Proteine und Lipoproteine. Hier wollen wir die Immunogenität und das Schutzpotenzial von oberflächenexponierten Pneumokokkenproteinen durch verschiedene in vitro und in vivo Studien entschlüsseln, um geeignete Impfstoffkandidaten zu identifizieren. Besonders Lipoproteine sind interessante Kandidaten, da sie hochkonserviert, reichlich vorhanden und immunogen sind.
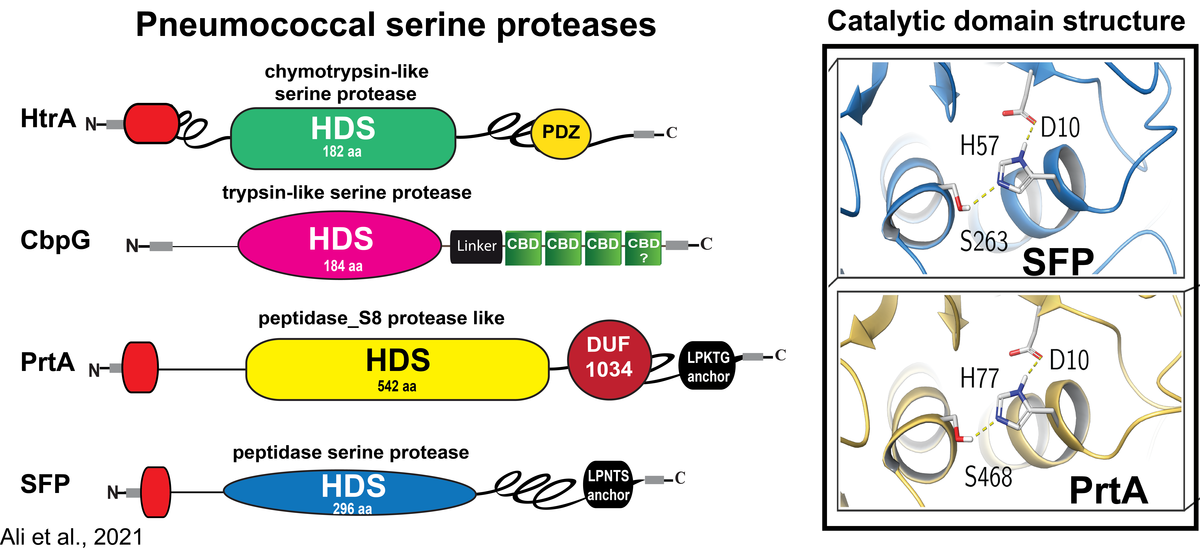
Rolle von Serinproteasen in der Pathophysiologie von Streptococcus pneumoniae
Pneumokokken besiedeln als harmlose Kommensalen die oberen Atemwege des Menschen. Bei der Übertragung in die Lunge können Pneumokokken unter bestimmten physiologischen und immunologischen Bedingungen die Epithelbarriere durchbrechen und Lungenentzündung sowie schwere invasive Erkrankungen verursachen. Wir und andere haben gezeigt, dass die Kolonisierung durch direkte Interaktionen mit Wirtszellrezeptoren oder durch Bindung an Komponenten der extrazellulären Matrix (ECM), die an Wirtszellrezeptoren gebunden sind, erleichtert wird. In ähnlicher Weise nutzen Pneumokokken die ECM- und Serumproteine, um dem angeborenen Immunsystem zu entgehen. Um die Epithelbarriere zu durchbrechen, missbrauchen Pneumokokken und andere Bakterien vom Wirt abgeleitete extrazelluläre Proteasen oder nutzen endogene Enzyme und oberflächenexponierte Proteasen für Gewebeumbauprozesse. Wir haben bereits gezeigt, dass Pneumokokken wirtsabgeleitete proteolytische Aktivitäten wie das Serinprotease-Plasmin(ogen) benutzen, um die extrazelluläre Matrix abzubauen, das Wirtsgewebe zu kolonisieren und letztlich einzudringen. Im Gegensatz dazu ist die Rolle von Pneumokokken-Serinproteasen, die die typische Asp-Ser-His-Triade enthalten, die für ihre katalytische Aktivität bei der Kolonisierung, dem Schleimabbau oder der Störung der Epithelbarriere unerlässlich ist, unzureichend erforscht.
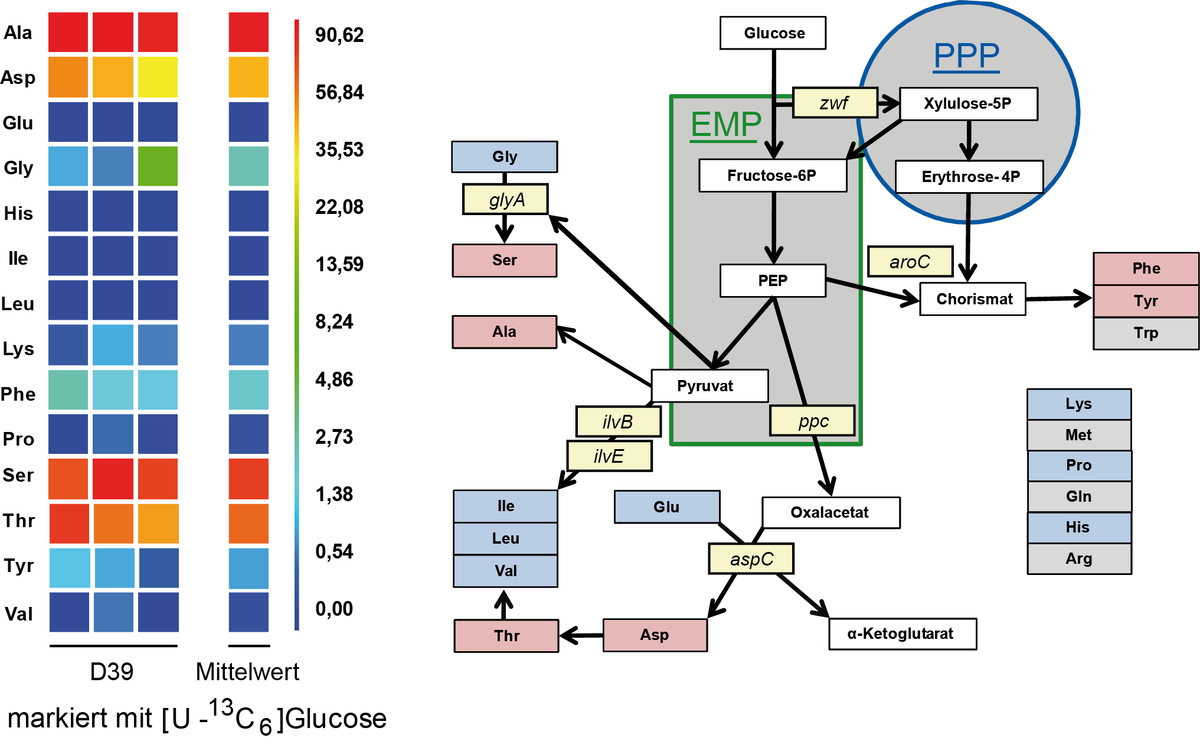
Studien zum Metabolismus in Streptococcus pneumoniae
Streptococcus pneumoniae kann verschiedene Zucker, wie Glukose, Galaktose oder auch Laktose als C-Quelle unter fakultativ anaeroben Bedingungen metabolisieren. Als Hauptendprodukt entsteht unter fermentativen Bedingungen Laktat. Galaktose ist zusätzlich ein wichtiger Baustein für die Kapsel Biosynthese. Der Einsatz von 13C markierter Glucose ermöglichte den Nachweis des Kohlenstoffmetabolismus mittels 13C Isotopenlogen Verteilung und GC/MS Analysen. Im Stamm D39 erfolgt dabei die Synthese von Serin aus Glycin durch die Serin Hydroxymethyltransferase. Der Organismus kann Glutamin nicht synthetisieren verfügt aber über 6 verschiedene Glutamin Transporter. Für weitere Studien werden 15N markierte Aminosäuren eingesetzt, deren Aufnahme studiert und deren Nutzung als wichtige Stickstoff Quelle analysiert werden soll.
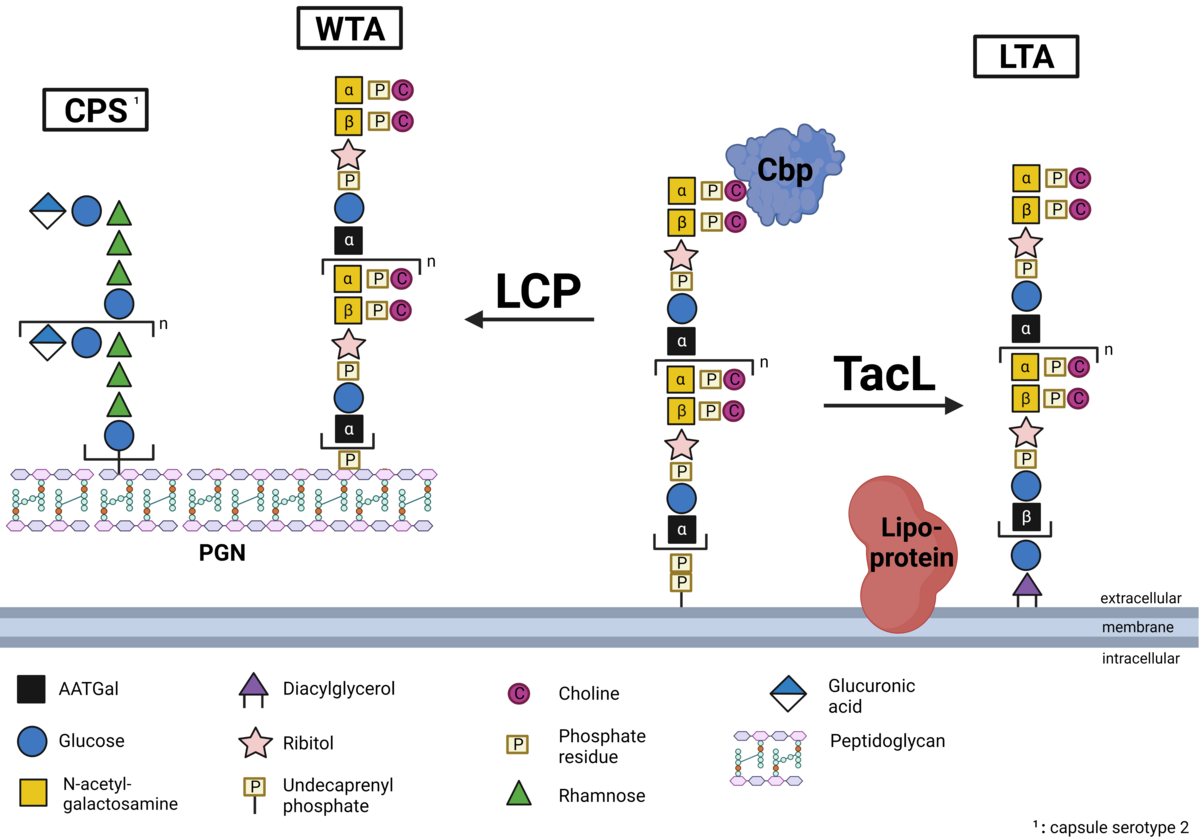
Biosynthese und Verankerung der Teichonsäuren bei Pneumokokken
Ein essentieller Bestandteil der Zellwand von Gram-positiven Bakterien und somit auch Streptococcus pneumoniae stellen die sogenannten Teichonsäuren dar. Es handelt sich dabei um Zuckerpolymere, die als Wandteichonsäure an das Peptidoglykan gebunden oder als Lipoteichonsäure in der Membran verankert sind. Pneumokokken-Teichosäuren sind mit Phosphorylcholin (PCho) substituiert und die so genannten Cholinbindungsproteine (CBPs) sind nicht-kovalent an die Teichosäuren gebunden. Während wir in S. pneumoniae die Verankerung von Lipoteichonsäuren in der bakteriellen Zellmembran durch die Teichonsäureligase (teichoic acid ligase, TacL) bereits aufklären konnten, konzentriert sich unsere laufende Forschung u.a. auf die Identifizierung der Proteine, die für die Verankerung von Wandteichosäuren im Peptidoglykan verantwortlich sind. Strukturchemische Analysen der bakteriellen Deletionsmutanten sowie die Quantifizierung der Teichonsäuren werden in Zusammenarbeit mit Dr. Nicolas Gisch aus dem Forschungszentrum Borstel, Leibniz Lungenzentrum, durchgeführt. Projekt-PI: Prof. Hammerschmidt, Doktorand: Max Brendel (Dipl.-Pharm), Co-Betreuung: Dr. Thomas Kohler (DFG HA 3125/5-2)
Aktuelle Drittmittel
- DFG | German Research Foundation
- BMBF | Federal Ministry of Education and Research - InfectControl 2020
- Federal State of Mecklenburg-Vorpommern | KoInfekt
- DAAD | German Academic Exchange Service
- IB-ASIEN | SOUTHEAST ASIA EUROPE
Kooperationen
- Prof. Dr. Juan Hermoso, CSIC, Madrid, Spanien
- Prof. Dr. Uwe Koedel, Neurologie, LMU München
- Prof. Dr. Manfred Rohde, Helmholtz-Zentrum für Infektionsforschung, Braunschweig
- Prof. Dr. Jan Maarten van Dijl, Groningen, Niederlande
- Prof. Dr. Marien DeJonge, Nijmegen, Niederlande
- Dr. Nicolas Gisch, Research Center Borstel
- Prof. Peter F. Zipfel, Hans-Knöll Institut, Jena
- PD Dr. Wolfgang Eisenreich, Biochemie, TU München
- Prof. Dr. Gustavo Gámez, Universidad de Antioquia, Medellín, Colombia
- Prof. Stefan Hippenstiel, Prof. Andreas Hocke, Prof. Leif Sander, Prof. Norbert Suttorp, Department of Internal Medicine, Infectious and Respiratory Diseases, Universitätsmedizin Berlin