The focus of the subject area is the analysis of physiological and pathological proteome profiles in human tissues, cells, biofluids or cellular and animal models. The main focus is on cardiovascular diseases, which are investigated within the framework of the German Centre for Cardiovascular Research (DZHK). In addition, oncological and developmental biological topics, among others, are investigated together with clinical research groups.
The aim is to identify biomarkers for the diagnosis of diseases or the prognosis of disease progression as well as risk factors and to better understand pathophysiological processes. For this purpose, methods in proteomics are adapted and corresponding evaluation strategies are established.
Principal Investigators
Research Assistants
Dr. Sabine Ameling
Dr. Manuela Gesell Salazar
Dr. Vishnu Mukund Dhople
Technical Assistance
Ulrike Lissner
Sophie Füsting
Specific research projects
Our network combines the expertise of basic medical research (University Medicine Greifswald), functional protein modeling (MIPS - Helmholtz Institute for Pharmaceutical Research Saarland), the multi-omics network analysis (Technical University of Munich) and the clinical application (University Medicine Greifswald).
Alternative splicing (AS) is a biological mechanism that allows to create different transcripts from the same DNA sequence. The overall goal of Sys_CARE is to investigate for the first time the importance of AS for the pathogenesis and the disease process with system medicine approaches and to develop the required bioinformatic methods.
To this end, we have access to a large dataset of molecular and clinical data, which will be complemented in the course of this project.
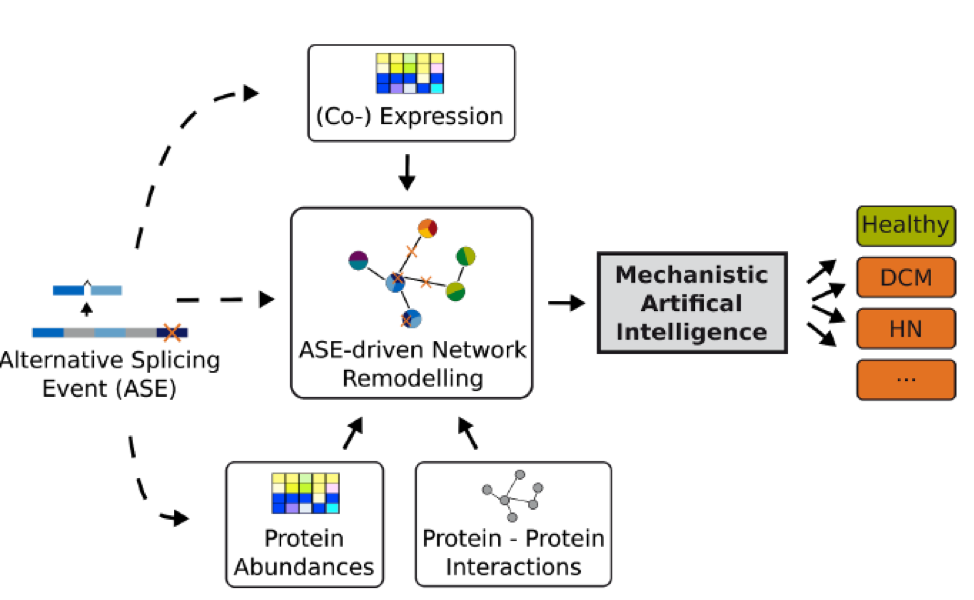
The focus is on the development of new methods that will allow previous molecular biology knowledge to be included into the analysis of highly complex data and thus enable the detection of disease-relevant changes in AS, which remain hidden with existing methods. Important sub-goals of this project are: First, the identification of transcripts which are more frequent or less common in patients than in healthy persons and that can potentially distinguish patients with dilated cardiomyopathy (DCM) and patients with hypertensive nephrosclerosis (HN). DCM is a condition in which the heart becomes enlarged and cannot pump blood effectively, while HN is an inflammatory kidney disease due to high blood pressure. Second, the elucidation of the role of miRNAs (short regulatory transcripts) for regulating AS and the investigation of the therapeutic potential of miRNAs to target disease-specific AS events.
In the course of the digital revolution, the use and processing of personal data has become important for the overall value chain. In this context, it is vital to ensure a balance of interests among all stakeholders. While business and research are interested in a smooth exchange of data, the people providing data want above all to preserve their data sovereignty in terms of data protection law and data ethics. One key to reducing the threat of asymmetries lies in the involvement of data trustees, i.e. neutral intermediaries.
In order to establish data trustees in the long term, a legal regulatory framework is needed that guarantees data protection and at the same time creates incentives to promote innovation. TreuMed is taking up this challenge. We develop and test data trustee models and corresponding business concepts using the example of "distributed artificial intelligence" in medicine. Machine learning methods in medical research rely on extensive, sensitive patient data to be able to make reliable predictions. However, strict data protection can counteract the success of research because it reduces the amount of data available.

This in turn has a negative impact on the success of patient treatment. TreuMed provides a technical solution to this dilemma with "distributed artificial intelligence" in medicine. The core of the TreuMed concept is a traffic light system for data trustees: depending on the identifiability of the patient data, one of three possible privacy levels and a corresponding level of protection is determined. The model is flanked by certification obligations and liability concepts for residual risks. Furthermore, the presented models are tested through applications of "distributed artificial intelligence" in the field of molecular epidemiology and biomarker research using the example of (identifying) genomics, transcriptomics and imaging data.
Within the framework of the German Center for Cardiovascular Diseases, protein, mRNA and miRNA analyses of patient samples are performed to contribute to a better understanding of pathomechanisms of cardiovascular diseases or of molecular changes during therapy. The focus is on dilated cardiomyopathy (DCM). This myocardial disease is characterized by a decrease in contractile function of the myocardium and by dilatation of the right and left ventricle.
Overall, however, the importance of heart failure (HI) with its etiologically very different causes is growing, and with it the need for better diagnostics and the development of improved therapeutic approaches. A prerequisite for this is the investigation of samples in existing and newly established patient cohorts for a better understanding of the molecular adaptations in HI.
Thus, the goal of this work is to detect differences in RNA and protein patterns in both tissues and biofluids of HI patients and control patients without impaired cardiac function by screening biospecimens. Diagnostic molecular signatures will be used to classify patient subgroups and enable therapy adjustments based on prediction of disease progression.
Collaboration partners will be supported in the molecular characterization of cellular and animal models of cardiovascular disease through protein and phosphopeptide profiling.
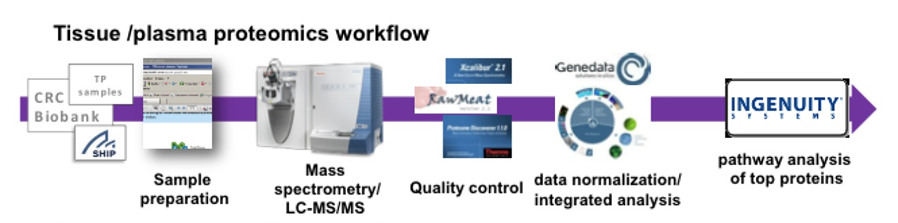
The development of individual therapy options is becoming increasingly important. OMICS technologies open up new possibilities for the identification of new biomarker candidates to support the diagnosis and prognosis of diseases and to derive individual therapy approaches. Our work focuses on profiling studies in the blood of subjects of the SHIP population study and patients with cardiovascular diseases. In addition to the necessary methods for profiling several hundred samples for miRNA by PCR and for proteins on the basis of the latest mass spectrometric technologies, workflows for standardisation, quality control as well as the individual but also integrated evaluation of data sets and their interpretation have been developed and can now be applied for the analysis of the most diverse phenotypes. The analysis portfolio is completed by the application of the methods to examinations of saliva and urine.
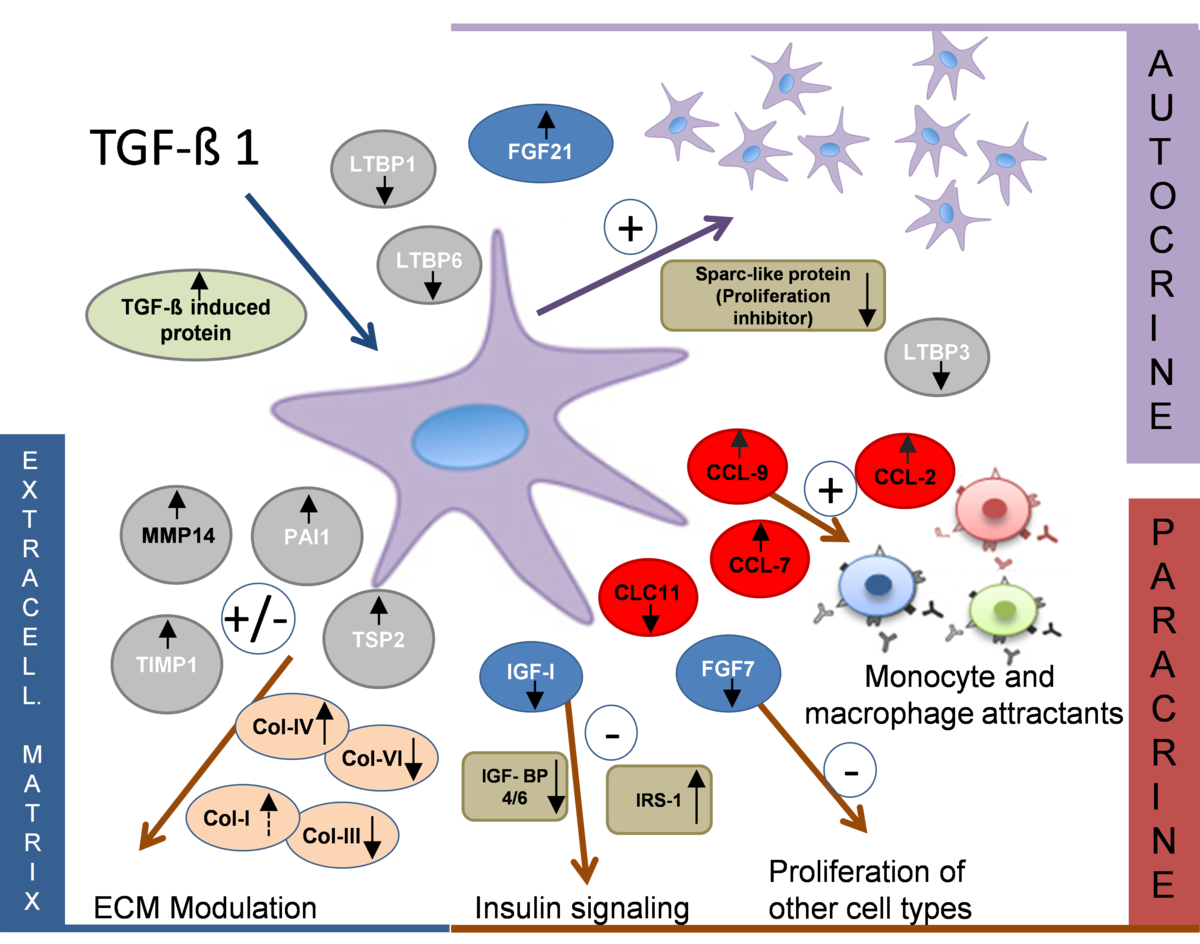
Cells secrete proteins that play an important role in maintaining cell integrity and stability, mobilisation and differentiation. Such proteins can act as autocrine (effect on cells of the same type) or paracrine (effect on other cell types) factors and indirectly initiate a cell response to external stimuli. The analysis of secreted proteins, also from cell cultures, requires special workflows that take into account the small amount of these protein species, their presence in low concentrations and interference with medium components. In the working group, adapted cell culture protocols and protein enrichment strategies have been established that enable a comprehensive mass spectrometric analysis of secretomes even from low cell numbers such as those present in stem cells.
Funding agencies
DZHK | German Centre for Cardiovascular Research
BMBF | Federal Ministry of Education and Research
DFG | German Research Foundation
Collaborations
Stephan Felix, Department for Internal Medicine B, University Medicine Greifswald
Jens Fielitz, Department for Internal Medicine B, University Medicine Greifswald
Thomas Kocher, Department of Restorative Dentistry, Periodontology, Endodontology, Preventive and Pediatric Dentistry, University Medicine Greifswald
Nicole Endlich, Department of Anatomy and Cell Biology, University Medicine Greifswald
Matthias Heckmann, Department of Neonatology and Pediatric Intensive Care, University Medicine Greifswald
Karin Klingel, Department of Molecular Pathology, University Hospital Tübingen, Germany
Dirk Westermann, University Heart Center, University Hospital Hamburg
Cristina Iuga, Department of Pharmaceutical Analysis, University of Medicine and Pharmacy "Iuliu Hatieganu", Cluj, Romania
Verena Stangl & Mario Lorenz, AG Lorenz/ Stangl, Charité Berlin