Siemens Lab - Infectious Diseases

Our lab explores the hidden battles between bacteria and the human immune system. We focus on how infections that often start harmless can turn severe, damaging tissues and overwhelming the body’s defenses. By uncovering these processes, we aim to understand why some infections become life-threatening and how we might prevent them.
Group leader
Nikolai Siemens
Dr. rer. nat., Professor
Nikolai received his diploma and doctoral degrees from the University of Rostock (Germany) in 2009 and 2012, respectively. He completed postdoctoral studies at the Center for Infectious Medicine, Karolinska Institutet (Stockholm, Sweden) in 2016. Nikolai established his own laboratory at the University of Greifswald in 2017. In 2021, he was appointed professor in molecular genetics of infections.
Contact:
Phone: +49 3834 420 5711
nikolai.siemensuni-greifswaldde

Administrator
| Nicole Born | Phone: +49 3834 420 5701 | E-Mail: nicole.born@uni-greifswald.de |
Group members
Anna Riegner
PhD student
Anna received her Master’s degree in Molecular Biology and Physiology from the University of Greifswald. In her PhD project, she studies how streptococci interact with the body’s early immune defenses. By uncovering how these first responses can sometimes make an infection much more serious, she aims to improve our understanding of how dangerous bacterial infections develop and how they might be better controlled in the future.
Contact:
Office +49 3834 420 5716
Lab +49 3834 420 5727
anna.riegner@uni-greifswald.de

Katharina E. Folz
PhD student
Katharina received her Master’s degree in Biochemistry from the University of Greifswald. In her research, she investigates how the immune system reacts to bacterial infections and how streptococci manage to evade these defenses in severe soft tissue infections. By uncovering the strategies these bacteria use to bypass immune protection, she aims to support a better understanding of life-threatening infections.
Contact:
Office +49 3834 420 5751
Lab +49 3834 420 5727
katharina.folzuni-greifswaldde

Vartika Singh
Wissenschaftliche Mitarbeiterin
Vartika received her Master’s degree in Plant Molecular Biology and Biotechnology from the University of Delhi. She supports the team in laboratory work and experimental activities, contributing to ongoing projects on streptococcal infections.
Contact:
Office +49 3834 420 5751
Lab +49 3834 420 5727
vartika.singhuni-greifswaldde

Constantin Klein
Technical assistant
Constantin completed his biology lab technician training at FLI Island Riems in 2020 and earned his Bachelor’s degree in Biology from the University of Greifswald in 2024. As a technical assistant in the group, he plays a key role in supporting experiments and maintaining laboratory operations, ensuring that research runs smoothly and efficiently.
Contact:
Phone +49 3834 420 5726
constantin.kleinuni-greifswaldde

Current BSc & MSc students
Jonathan Hamann (BSc student)
s-johamauni-greifswaldde
Sandro Urtecho Valverde (BSc student)
s-saurteuni-greifswaldde
Chelcie M. Djong (MSc student)
s-chdjonuni-greifswaldde

Projects
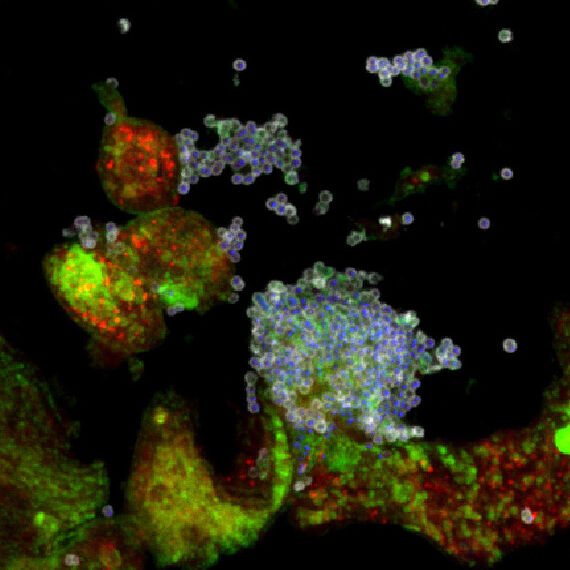
Some bacteria that usually cause mild infections can suddenly become dangerous. Group A, B, C and G streptococci, and Staphylococcus aureus are well known for this behavior. They may cause a sore throat or a small skin infection in one situation, yet in another they can trigger sepsis or rapidly spreading soft tissue infections.
The key to this change lies in their interaction with the body’s first line of defense. Innate immune cells, keratinocytes and fibroblasts release signals that can activate molecular switches inside the bacteria. These switches influence whether bacteria hide, persist or become far more aggressive (1, 2).
We investigate these crucial host pathogen interactions to understand what drives this shift. By examining how human cells respond and how bacteria adapt in return, we aim to identify the events that push an infection toward severe disease. This knowledge will support the development of better ways to detect and manage these dangerous infections.
Main experimentator(s): Anna Riegner & Katharina E. Folz | Funding: DFG (German Research Foundation)
This research is performed with a number of international and national collaborators, including Anna Norrby-Teglund (Karolinska Institutet, Stockholm, Sweden).

Streptococci are responsible for a wide range of diseases, including life-threatening necrotizing skin and soft tissue infections. While biofilms have long been associated with chronic infections or foreign devices, their role in acute streptococcal infections has only recently come into focus. In our studies, we discovered dense, multicellular biofilm communities directly in the skin tissue of infected patients (3, 4).
These biofilms are highly diverse, with different bacterial subpopulations exhibiting distinct behaviors. Some cells appear dormant, while others are more active and can either stay within the biofilm or disperse into surrounding tissue, potentially driving the spread of infection.
Our research aims to uncover how these biofilm communities function and communicate. By exploring the metabolic activity and signaling pathways of the different bacterial groups, we hope to reveal the strategies streptococci use to persist, adapt, and evade the host. Understanding these mechanisms could open new avenues for therapies that prevent bacterial spread and improve outcomes for patients with severe infections.
Main experimentators: Katharina E. Folz & Constantin Klein | Funding: DFG (German Research Foundation)
This research is performed in close collaboration with Michael Lalk´s group (Institute of Biochemistry, Greifswald) and international collaborators: Bård R. Kittang, Oddvar Oppegaard, Steinar Skrede (Haukeland University Hospital, Bergen, Norway), Anna Norrby-Teglund (Karolinska Institutet, Stockholm, Sweden).

Our body relies on a frontline army of cells to fight bacterial invaders. Neutrophils rush to infection sites to engulf bacteria, release antimicrobial molecules, and form traps that capture microbes. Monocytes circulate in the blood and can become macrophages, coordinating immune responses and alerting other cells. Macrophages patrol tissues to clear pathogens and debris, while dendritic cells present bacterial antigens to activate the wider immune system. Platelets, best known for clotting, also influence immune cell activity and bacterial survival.
Bacteria, such as streptococci and staphylococci, interact closely with these cells, altering immune cell function, while immune cells in turn control bacterial survival, persistence, and spread.
Our research explores these dynamic host–pathogen interactions to understand what drives infections to become severe. By revealing how bacteria and immune cells influence each other, we aim to identify new strategies to protect tissue and improve patient outcomes (5,6,7,8).
Main experimentator(s): Anna Riegner & Katharina E. Folz | Funding: DFG (German Research Foundation)
Key collaborators
- Anna Norrby-Teglund, Karolinska Institutet, Stockholm, Sweden
- Steinar Skrede, University of Bergen, Bergen, Norway
- Lakshmi Rajagopal, University of Washington, Seattle, WA, USA
- Natalia Korotkova, University of Kentucky, Lexington, KY, USA
- John McCormick, Western University, London, Ontario, Canada
- Marcus Fulde, FU Berlin, Berlin, Germany
- Sonja Oehmcke-Hecht, University Medicine Rostock, Rostock, Gremany
- Bernd Kreikemeyer, University Medicine Rostock, Rostock, Germany
- Michael Lalk, University of Greifswald
- Sven Hammerschmidt, University of Greifswald
- Uwe Völker, University Medicine Greifswald
- Thomas Thiele, University Medicine Greifswald
- Katharina Hoff, University of Greifswald
- Janosch Schoon, University Medicine Greifswald
- Georgi I. Wassilew, University Medicine Greifswald
Publications
A validated model for early prediction of group A streptococcal aetiology in necrotising soft tissue infections using minimal patient data. Katz S, Suijker J, Skrede S, Meij-de Vries A, Pijpe A, Norrby-Teglund A, Medina LMP, Damås JK, Hyldegaard O, Solligård E, Svensson M; PerAID/PerMIT/INFECT study group¶; Mosevoll KA, Dos Santos VAPM, Saccenti E. BMC Medicine. 2026 Jan 10. doi: 10.1186/s12916-025-04593-y. ¶ member of the study group
All publications:PubMedGoogle Scholar
Selected publications:
Streptococcus pyogenes activates human platelets via streptolysin S-mediated calcium ion influx. Riegner A, Jahn K, Wesche J, Thiele T, Siemens N. The Journal of Innate Immunity. 2025. Mar 3:1-24. doi: 10.1159/000544951.
Streptokinase is dispensable in Streptococcus dysgalactiae subspecies equisimilis infections of human dendritic cells. Folz KE & Siemens N. Scientific Reports. 2025 Jan 21;15(1):2723. doi: 10.1038/s41598-025-87404-x
Streptokinase reduces Streptococcus dysgalactiae subsp. equisimilis biofilm formation. Tölken LA, Neufend JV, Oppegaard O, Methling M, Moll K, Redanz S, Katsburg MMD, Ali MQ, Shumba P, Kreikemeyer B, Skrede S, Fulde M, Norrby-Teglund A, Lalk M, Kittang BR, Siemens N. BMC Microbiology. 2024 Sep 30;24(1):378. doi: 10.1186/s12866-024-03540-w.
Reduced interleukin-18 secretion by human monocytic cells in response to infections with hyper-virulent Streptococcus pyogenes. Tölken LA, Paulikat AD, Jachmann LH, Reder A, Gesell Salazar M, Palma Medina LM, Michalik S, Völker U, Svensson M, Norrby-Teglund A, Hoff KJ, Lammers M, Siemens N. Journal of Biomedical Science. 2024 Feb 27;31(1):26. doi: 10.1186/s12929-024-01014-9.
Streptococcus pyogenes reversibly abrogates SpeB secretion in response to neutrophil-derived reactive agents in tissue infections. Shumba P, Sura T, Moll K, Chakrakodi B, Tölken LA, Hoßmann J, Hoff KJ, Hyldegaard O, Nekludov M, Svensson M, Arnell P, Skrede S, INFECT Study Group, Norrby-Teglund A, and Siemens N. Journal of Biomedical Science. 2023 Jul 10;30(1):52. doi: 10.1186/s12929-023-00947-x.
Bronchial Epithelial Cells Accumulate Citrate Intracellularly in Response to Pneumococcal Hydrogen Peroxide. Surabhi S, Jachmann LH, Lalk M, Hammerschmidt S, Methling K, Siemens N. ACS Infectious Diseases. 2021 Oct 8. doi: 10.1021/acsinfecdis.1c00372.
Hydrogen Peroxide is Crucial for NLRP3 Inflammasome-Mediated IL-1β Production and Cell Death in Pneumococcal Infections of Bronchial Epithelial Cells. Surabhi S, Jachmann LH, Shumba P, Burchhardt G, Hammerschmidt S, Siemens N. The Journal of Innate Immunity. 2021. Aug 6:1-15. doi: 10.1159/000517855
Pathogenic Mechanisms of Streptococcal Necrotizing Soft Tissue Infections. Siemens N, Snäll J, Svensson M, Norrby-Teglund A. Advances in Experimental Medicine and Biology. 2020;1294:127-150. doi: 10.1007/978-3-030-57616-5_9.
Adenosine Triphosphate Neutralizes Pneumolysin-induced Neutrophil Activation. Cuypers F, Klabunde B, Gesell Salazar M, Surabhi S, Skorka SB, Burchhardt G, Michalik S, Thiele T, Rohde M, Völker U, Hammerschmidt S, Siemens N. The Journal of Infectious Diseases. 2020. Oct 13;222(10):1702-1712. doi: 10.1093/infdis/jiaa277.
Is it time to reconsider the group A streptococcal rheumatogenic concept? Norrby-Teglund A and Siemens N. Clinical Infectious Diseases. 2020 Mar 17;70(7):1461-1462. doi: 10.1093/cid/ciz427.
Shocking superantigens promote establishment of bacterial infection. Siemens N and Norrby-Teglund A. Proc Natl Acad Sci USA. 2017 Sep 19;114(38):10000-10002. doi: 10.1073/pnas.1713451114. Epub 2017 Sep 12.
Biofilm in group A streptococcal necrotizing soft tissue infections. Siemens N, Chakrakodi B, Shambat SM, Morgan M, Bergsten H, Hyldegaard O, Skrede S, Arnell P, Madsen MB, Johansson L; INFECT Study Group, Juarez J, Bosnjak L, Mörgelin M, Svensson M, Norrby-Teglund A. Journal of Clinical Investigation Insight. 2016 Jul 7;1(10):e87882. doi: 10.1172/jci.insight.87882.
Former group members
Dr. Lea A. Tölken,Thesis: Myeloid cells' response to infection with Staphylococcus aureus and Streptococcus pyogenes (2024)
Dr. Patience Shumba,Thesis: The role of secreted virulence factors in disease progression and severity during streptococcal and pneumococcal infections (2022)
Dr. Surabhi Surabhi,Thesis: The Role of Hydrogen Peroxide and Pneumolysin in Pneumococcal Pathogenesis (2021)
Dr. Fabian Cuypers, Thesis: Responses of the innate immune axis in respiratory infections (2021)
Dr. Antje D. Paulikat,Thesis: Impact of respiratory tract pathogens on the function of innate immune cells (2021)
Katharina Meyer (BSc Human Biology, 2025)
Jacqueline Mucks (MSc Molecular Biology and Physiology, 2025)
Tabea Tzschapke (BSc Biology, 2025)
Katharina Folz (MSc Biochemistry, 2024)
Debby Schubert (BSc Biology, 2024)
Emily Friedrich (MSc Human Biology, 2024)
Carolina Wieters (BSc Biology, 2024)
Max Sittner (MSc Human Biology, 2024)
Anna Riegner (MSc Molecular Biology and Physiology, 2022)
Celina Würner (MSc Biochemistry, 2022)
Open positions
We are always interested in connecting with talented and highly motivated researchers who share our passion for translational research on the mechanisms underlying highly lethal infectious diseases. If you would like to pursue a research project within our group, please contact the group leader, Nikolai Siemens.
Teaching
5102302 VC2 Lecture: Molecular Genetics of Eukaryotes
5102308 VC2 Lecture: Molecular Biotechnology of Eukaryotes (is replaced since WiSe 2024/25!)
5102402 VC2 Practical Course: Genetics II
Contribution to:
5102350 CM1 Lecture Series; 5102351 CM1 Seminar Series; 5102430 CM1 Practical Course; 5102043 F5 Practical Course: Basics in Genetics
5102353 AM2 Lecture: Host and Pathogen Genetics in Infectious Diseases
5102354 AM2 Seminar: Host and Pathogen Genetics in Infectious Diseases
5102431 AM2 Practical Course: Host and Pathogen Genetics in Infectious Diseases
5102030 F5 Lecture: Biotechnology
